What Is Molar Pregnancy?
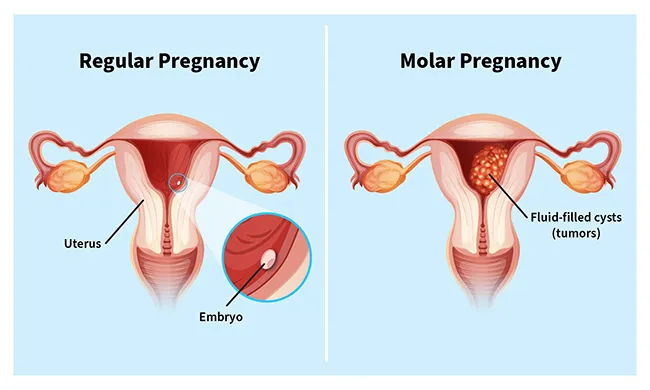
A molar pregnancy is a complication in which an abnormal mass or tumor forms inside your uterus, instead of a placenta. In a healthy pregnancy, the placenta is an organ that grows in the uterus and nourishes a developing baby through the umbilical cord.
With a molar pregnancy, the embryo can't develop. This results in the loss of the pregnancy, usually through a miscarriage.
Molar pregnancy is a type of gestational trophoblastic disease (GTD). These are rare conditions in which tumors grow in your uterus during early pregnancy. They stem from abnormal changes in the trophoblast, a layer of cells that surrounds an embryo shortly after conception.
Your doctor might call a molar pregnancy a hydatidiform mole (HM).
Types of Molar Pregnancy
There are two types of molar pregnancy, partial and complete.
Partial molar pregnancy
In a partial molar pregnancy, both the placenta and embryo (fertilized egg) are abnormal. It usually happens after an egg is fertilized by two sperm. A fetus may develop, but it can't survive. This type is sometimes called an incomplete molar pregnancy.
Complete molar pregnancy
In a complete molar pregnancy, there’s an abnormal placenta, but no embryo. This happens after a sperm fertilizes an egg that doesn't contain any genetic material. This type is more common than the partial type.
Molar Pregnancy Causes
Molar pregnancies are caused by genetic problems that happen during conception. Scientists don't know exactly what causes them.
Normal human cells contain 23 pairs of chromosomes, one set from each parent. These structures carry information that tells the body’s cells what to do.
In a complete molar pregnancy, the egg doesn't contain any chromosomes, which results in the absence of half of the genetic material.
In a partial molar pregnancy, there’s an extra set of chromosomes that comes from the father. When this happens, the fertilized egg can’t survive. It usually dies a few weeks into the pregnancy.
Molar Pregnancy Risk Factors
In the U.S., 1 out of every 1,000 pregnancies are molar pregnancies. Things that may increase your chances include:
- Being younger than 20 or older than 40
- Having experienced a molar pregnancy in the past
- Having a history of pregnancy loss
- Difficulty conceiving or infertility problems
- Being of Asian descent
Molar Pregnancy Symptoms
Sometimes, molar pregnancy doesn't cause any symptoms. But they can include:
- Bleeding from your vagina in the first 3 months of pregnancy
- Watery brown discharge
- Sacs (they look like clusters of grapes) that pass out of your vagina
- Nausea and vomiting that happen more often or are worse than what’s normal during pregnancy
- Lots of pressure or pain in your pelvis
- More swelling in the belly area than expected for your stage of pregnancy
- Preeclampsia (high blood pressure)
- Anemia, in which your body has too few red blood cells
- Very high levels of the pregnancy hormone human chorionic gonadotropin (hCG)
- Cysts on your ovaries
- An overactive thyroid (hyperthyroidism)
Call your doctor if you notice any unusual symptoms during pregnancy.
Molar Pregnancy Diagnosis
You might find out you have a molar pregnancy during a routineultrasound test. Your doctor could detect a problem through a blood test that shows your hCG levels are much higher than they should be. Or you might not know until you have a miscarriage.
Molar pregnancy ultrasound
An ultrasound is a device that uses sound waves to look inside your uterus. You might have a transvaginal ultrasound, in which your doctor places a wandlike device in your vagina to create the image.
If you have a partial molar pregnancy, the ultrasound might show:
- Little amniotic fluid
- A fetus that's smaller than it should be
- A placenta that looks unusual
With a complete molar pregnancy, your doctor might see
- No amniotic fluid
- No embryo
- A placenta that's very large and thick
- Cysts on your ovaries
If your doctor finds you have a molar pregnancy, they may do further imaging tests, such as a CT scan or MRI, to see if the tumor has spread to other parts of your body.
Molar Pregnancy Treatment
Molar pregnancy treatments involve removing the placental tissue to prevent complications.
Your doctor may use a procedure called dilation and curettage (D&C). This is often the treatment for pregnancy loss due to any reason. During this procedure, your doctor opens your cervix with special tools and removes the tissue from your uterus.
Sometimes, doctors use medication for this purpose. It causes the uterus to contract and expel its contents.
If you don’t want to get pregnant again, you may consider a total hysterectomy. That’s surgery to remove your uterus.
If your blood type is Rh-negative, you'll get a shot of the drug Rh immunoglobulin as part of your treatment for molar pregnancy. This helps prevent complications.
After the molar tissue is removed, your doctor may do blood tests several times over the next few months to see whether your hCG levels are getting back to normal.
Molar Pregnancy Complications
Persistent gestational trophoblastic neoplasia
In rare cases, tissue remains after a molar pregnancy is removed or miscarried. The abnormal tissue may grow outside your uterus and into the layer of muscle below it. This condition is called persistent gestational trophoblastic neoplasia (GTN). It's more likely with complete molar pregnancies.
To treat persistent GTN, you may need to receive chemotherapy (“chemo”) or have a hysterectomy.
Choriocarcinoma
A molar pregnancy can also lead to a type of cancer called choriocarcinoma, which can spread to other areas of your body. This is rare but more likely to happen with a complete molar pregnancy than a partial one. Your doctor can treat choriocarcinoma with chemo or radiation.
Other complications
Some other complications are possible, including:
- Preeclampsia (high blood pressure)
- Shock (low blood pressure)
- Infection in your uterus
- Blood infection (sepsis)
Does a molar pregnancy cause infertility?
Molar pregnancy doesn't affect your fertility. But after a molar pregnancy, your doctor might recommend that you don’t conceive for up to a year. Pregnancy increases hCG levels, so it would be hard for them to know whether the rise in hormones is due to that, abnormal tissue that’s still in your body, or choriocarcinoma.
Molar Pregnancy Prevention
The only way to be certain you won’t have a molar pregnancy is not to get pregnant. If you’ve had a molar pregnancy in the past, talk to your doctor. Ask them about the chances of it happening again. And find out how you’ll be monitored if you do get pregnant.
Takeaways
Molar pregnancy is when an abnormal clump of cells develops in your uterus, rather than a placenta. This means the embryo can't develop. Molar pregnancy often ends in miscarriage. But if that doesn't happen, it requires treatment to avoid complications.
Molar Pregnancy FAQs
How serious is a molar pregnancy?
If you miscarry a molar pregnancy or have been successfully treated for one, you're not likely to have serious issues. Rarely, you could develop serious complications, including a type of cancer called choriocarcinoma. But the complications can often be successfully treated.
Can you still have a baby with a molar pregnancy?
A molar pregnancy cannot develop into a baby. But having a molar pregnancy doesn't prevent you from conceiving and having a healthy pregnancy in the future.
How long can you carry a molar pregnancy?
If you have a partial molar pregnancy, the embryo can generally survive no longer than 3 months. If you don't have a miscarriage, your doctor will need to do a procedure or give you medication to remove it from your body. Prompt treatment is important to avoid complications.


