
You are correct!

Incorrect
The correct answer is: Testicular
The U.S. isn't in the lead, but it is right up there -- we come in third with 3,680 calories consumed each day.
More than a third of Americans are obese, suggesting that diet and exercise alone aren't enough for millions of people trying to slim down.
But they're not exactly clamoring for medical treatments for their disease, leaving a large and relatively untapped market for a number of new approaches that are in the pipeline.
Weight loss surgery is highly effective, but less than 2% of people who are candidates for it take that step, according to one estimate. "Patients are very reluctant to undergo surgery," says Caroline Apovian, the director of the Nutrition and Weight Management Center at Boston Medical Center. "They just don't realize that their obesity is not under their control, that it's a disease."
In January, the FDA approved the MAESTRO System, a pacemaker-like device designed to control hunger and fullness by blocking the vagus nerve, which extends from your brain to your stomach. The system, called the VBLOC, is one of a few intriguing approaches that researchers are studying as potential obesity treatments. VBLOC doesn't alter the stomach or intestine like many of the weight loss surgeries.
The devices represent a field energized by recent FDA approvals of four new obesity drugs: Qsymia in 2012, Belviq in 2013, Contrave this past September, and Saxenda in late December.
Sales of those treatments haven't taken off, though, in part because some people might not like the side effects, and some of the drugs aren't covered by insurance.
The history of failed obesity medications seems to have dampened people's enthusiasm for taking pills to help control their weight, says Harold Bays, MD. He's the medical director and president of the Louisville Metabolic and Atherosclerosis Research Center. "The problem is almost all of them ended up having bad side effects," Bays says.
Still, researchers continue to look for safer drugs, as well as for devices that might be more attractive than surgery.
These are some of the potential new treatments:
The ReShape Duo, a double balloon that is filled with saline and placed in the stomach for 6 months, has been on the market in Europe since 2007. It's intended to help people feel full, and it's under FDA review.
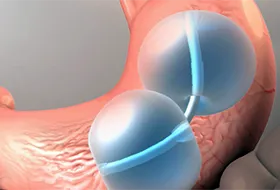
In a 6-month clinical trial of the device in obese patients in the U.S., more people who received the Reshape Duo device lost weight than those treated with counseling alone. The results were presented last November at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Nearly all patients had nausea, vomiting, and abdominal discomfort in the first few days. The symptoms went away in about a week, says Jaime Ponce, MD, a weight loss surgeon who led the study.
Gelesis100 is a cross between a pill and a device. It's a capsule filled with tiny particles created with raw materials used in food products.
The particles soak up water and expand in the stomach. They also mix with digested food and slow its passage out of the stomach, the company says. In a 12-week study reported at a medical conference last June, people on Gelesis100 lost 6.1% of their weight, compared to a 4.1% loss in a group of people who received a fake treatment.
The Elipse balloon, another device, has been revised several times. The researchers' goal was to make a balloon that could be placed in the stomach and removed without endoscopy, a procedure which usually requires sedation and a specially trained doctor.
Their latest model is attached to a long, thin, flexible tube. You swallow the balloon, which is then filled with about 15 ounces of water. The tube is then pulled out.
In its first human test, the balloon remained full for 6 weeks in six of eight people, researchers reported at last year's metabolic and bariatric surgery society meeting. Then it emptied and passed out of the body. Although the participants weren't prescribed diet or exercise, they lost 5 pounds on average. The balloon's maker, Allurion Technologies, is now doing a clinical trial in Europe, where it hopes to launch Elipse by early 2016.
The Obalon balloon works a little differently to help you feel full. First, you swallow a capsule that contains one balloon. The capsule dissolves and releases the balloon, which your doctor inflates through a tube attached to the capsule. The doctor then removes the tube. The doctor can place up to 3 balloons during the 12-week treatment. Then they are removed. The device is approved in several countries in Europe and in Mexico. In January, the company announced it had raised money to begin clinical trials in the U.S. In small studies, patients loss an average of 50% of their excess weight and 8% of their body weight, according to data provided by the company.
EndoBarrier is a thin, flexible liner placed at the beginning of the intestine. It's left in place for a year before being removed. Because it blocks food from a portion of the intestinal wall, it's supposed to alter the release of hormones that play a role in digestion.
Although it's not available in the U.S., it is on the market in several other countries. It's intended to treat obese people with a body mass index (BMI) of 35 or more, or those with a BMI of 30 or more with type 2 diabetes or at least one other obesity-related condition.
In the U.S., EndoBarrier is being studied to treat type 2 diabetes in obese people, maker GI Dynamics said in a statement.
Beloranib "allows the patient to burn off stored fat without feeling hungry," says Thomas Hughes, PhD. He's the CEO of Zafgen, the Boston company that developed the drug. The first drug of its type, beloranib is given in twice weekly injections.
Weight loss drugs already on the market "do not address the underlying problem in obesity," which is the way obese people metabolize fat, Hughes says. Trials of beloranib have shown that patients convert less of the calories they're eating into stored fat, and it stimulates the burning of fat that's already stored, he says.
Zafgen is seeking approval for beloranib for three conditions: Prader-Willi syndrome, a genetic disorder that causes unstoppable hunger and profound obesity; "acquired Prader-Willi syndrome," which results from the treatment of benign tumors in the middle of the brain; and severe obesity complicated by type 2 diabetes.
Mirabegron, approved by the FDA in 2012 as Myrbetriq to treat overactive bladder, might also help people lose weight. It works by activating a protein on bladder cells, which also is found on fat cells.
A study funded by the National Institutes of Health tested whether mirabegron could activate brown fat, a type of body fat that creates heat by burning calories.
A single high dose raised the metabolic rate -- the amount of energy the body burns at rest -- in 12 thin young men by an average of 13%, researchers reported Jan. 6 in Cell Metabolism.
"We don't know yet what to expect on metabolism in non-lean people," says Aaron Cypess, MD, PhD, of the National Institute of Diabetes and Digestive and Kidney Diseases.
He is planning longer-term studies of mirabegron in more diverse groups, including women and obese people.


The correct answer is: Testicular
The U.S. isn't in the lead, but it is right up there -- we come in third with 3,680 calories consumed each day.
Patients ... just don't realize that their obesity is not under their control, that it's a disease."