Migraine is a brain and nervous system disorder whose symptoms almost always include intense headaches. You get these headaches repeatedly, in episodes that can last anywhere from 4 to 72 hours. Along with head pain, they include other symptoms like nausea and sensitivity to light.
Migraine vs. headache
Many people use the word "migraine" to describe the headache itself. But head pain is just one symptom of migraine. And headaches can be caused by many other things. Some typical characteristics of migraine headaches include:
- The pain is throbbing or pounding and feels worse when you move around.
- You feel it mostly on one side of your head.
- You also have at least one of these symptoms: sensitivity to light and/or sound, nausea, and vomiting.
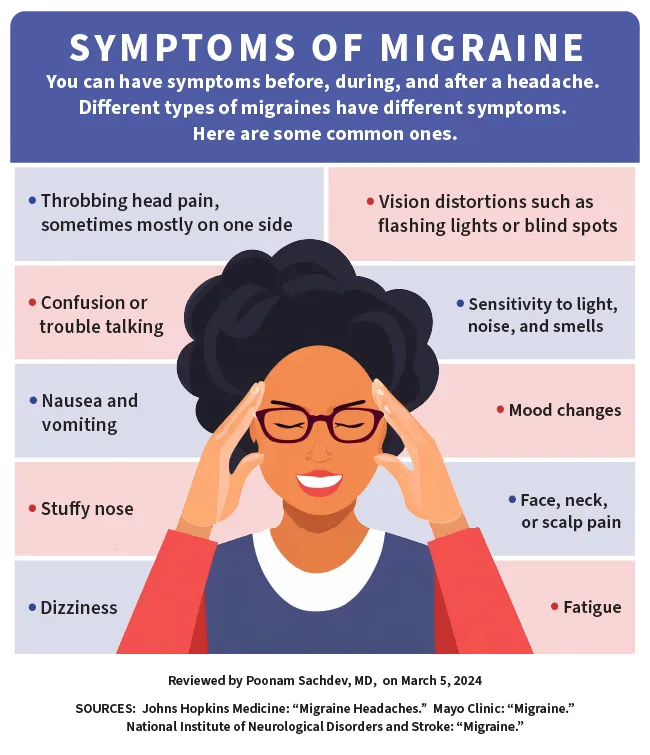
Migraine Symptoms
For many people, a migraine episode happens in stages. Symptoms you may have during these stagesinclude:
Migraine prodrome
Hours or days before a headache, about 60% of people who have migraine notice symptoms like:
- Sensitivity to light, sound, or smell. Levels of light and sound that don't usually bother you may feel uncomfortable or even painful. Odors like those of cigarette smoke, car exhaust, perfume, and cleaning products may seem especially unpleasant.
- Fatigue for no known reason. You might also yawn a lot.
- Food cravings or lack of appetite . You may crave a particular food like sweets or feel hungrier in general. Or you might not feel much like eating.
- Mood changes, such as irritability or sadness.
- Feeling thirstyand peeing more often.
- Digestive problems like feeling bloated or having constipation or diarrhea.
Experts say it's possible to mistake symptoms of prodrome for things that trigger an attack. For example, a craving for sweets might lead you to eat chocolate, which you then blame for causing your headache.
Migraine aura
Up to 25% of people with migraine have what's called auras shortly before after a headache starts or at the same time as a headache. These symptoms stem from your nervous system and often involve your vision. They usually start gradually, over a 5- to 20-minute period and last less than an hour. You may have:
- Vision problems like black dots, wavy lines, flashes of light, or tunnel vision
- Tingling or numbness in your face, hands, and/or limbs
- Trouble speaking clearly.You might mumble or slur your words.
- Ringing in your ears, called tinnitus
- Weaknesson one side of your face or body
Migraine attack
Also called the headache phase, this stage is usually characterized by head pain. This pain may:
- Begin as a dull ache and then become throbbing
- Get worse during physical activity
- Affect one side of your head or move from one side to the other, be in the front of your head, or affect your entire head
During the headache, you may also:
- Have nausea (About 80% of people with migraine do, and about half vomit.)
- Be paleand clammy
- Feel faintor dizzy
- Have neck pain or stiffness
- Feel anxious or depressed
- Have a runny nose or feel stuffed up
- Be sensitive to light(Noise or odors might also bother you.)
- Have trouble sleeping and feel fatigued
How long do migraines last? Often, it's about 4 hours, but serious ones can go for more than 3 days. Some people get them almost every day, while others get them once or twice a year.
Migraine postdrome
This stage can last up to a day after a headache. About 80% of people with migraine have postdrome. Symptoms include:
- Feeling tired, wiped out, or cranky
- Feeling unusually refreshed or happy
- Muscle pain or weakness
- Food cravings or lack of appetite
- Trouble concentrating
Migraine vs. tension headache
Tension headaches, often caused by tight muscles or stress, are the most common type of headache. The pain of a tension headache usually affects both sides of your head and feels steady instead of throbbing. Tension headaches don't usually cause nausea, vomiting, or light sensitivity.
Migraine vs. cluster headache
Cluster headaches tend to begin quickly with no warning. The pain is intense and sharp or burning. You usually feel it around or behind one of your eyes. Cluster headaches can cause these symptoms in the affected eye:
- Redness or watering
- An eyelid that looks droopy or swollen
- A smaller pupil
Each headache lasts between 15 minutes and 3 hours, and you can have several in 1 day. You tend to get them in "clusters"—weeks or months in which you have them often. These periods are followed by weeks or months in which you're headache-free. Cluster headaches aren't considered migraines.
Migraine Causes
Doctors don’t know exactly what causes migraines, though they seem to be related to your genes, as well as to changes in your brain. Your genes may even influence what triggers your headaches, whether it's fatigue, bright lights, or weather changes.
For many years, scientists thought people got migraine attacks because of changes in blood flow in the brain. Most now think this contributes to the pain but isn't what starts it.
Some experts now believe an attack starts due to chemical changes in your brain. Overactive nerve cells send out signals that lead to changes in the levels of certain chemical compounds in your body, such as serotonin and calcitonin gene-related peptide (CGRP). CGRP makes blood vessels around your brain swell, while serotonin causes them to shrink. An imbalance in these chemicals causes inflammation and pain.
Migraine Risk Factors
The American Migraine Foundation estimates that more than 38 million Americans get migraines. Some things may make you more likely to get them:
Sex. Women (and those assigned female at birth) have migraines three times more often than men and those assigned male at birth.
Age. Most people start having migraine headaches between ages 10 and 40. If you have periods, you may find that your attacks get better or go away after menopause.
Family history. Four out of five people with migraine have other family members who get them. If one parent has a history of these types of headaches, you have a 50% chance of getting them. If both parents have them, the risk jumps to 75%.
Other medical conditions. Depression, anxiety, bipolar disorder, sleep disorders, and epilepsy can raise your odds.
Migraine Triggers
Some things that may trigger an attack include:
Hormone changes. You maynotice that you have headaches around your period, while you're pregnant, or when you're ovulating. Symptoms may also be tied to menopause, hormonal birth control methods, or hormone replacement therapy.
Stress. When you're stressed, your brain releases chemicals that cause blood vessel changes that might lead to a headache.
Skipping meals
Changes in weather. Storm fronts, changes in barometric pressure, strong winds, or changes in altitude can all trigger an attack.
Your senses. Loud noises, bright lights, and strong smells can set off a headache.
Medications. Vasodilators, which widen your blood vessels, can trigger headaches.
Physical activity. This includes exercise and sex.
Tobacco
Changes in your sleep. You might get headaches when you sleep too much or not enough.
Migraine trigger foods
Some foods and drinks, such as aged cheeses, alcohol, chocolate, and beans, have been linked to migraine attacks. So have food additives like nitrates (in pepperoni, hot dogs, and lunch meats), monosodium glutamate, and the artificial sweetener aspartame. There's little solid research on this, so your best bet may be to keep a food diary to see whether any foods or drinks seem to trigger your headaches.
Foods or drinks that contain caffeine, such as coffee and tea, can trigger headaches for some people. Either having too much caffeine or not having as much as you're used to can cause an attack. Caffeine itself can be a treatment for headaches.
Migraine Types
There are several types of migraines. The most common are migraine with aura (also known as classic migraine) and migraine without aura (or common migraine).
Others include:
Menstrual migraine. This is when your headaches are linked to your period. They usually start 2 days before your period and last until 3 days after. You may also have other kinds of migraine headaches at other times of the month. Menstrual migraines don't usually include aura.
Silent migraine. With this kind, also known as an acephalgic migraine, you have aurasymptoms without a headache. You may also have nausea and other migraine symptoms. An attack usually lasts only about 20-30 minutes.
Vestibular migraine. You have balance problems, vertigo, nausea, and vomiting, with or without a headache. It usually happens in people who have a history of motion sickness.
Abdominal migraine. Experts don't know a lot about this type. It causes stomach pain, nausea, and vomiting. It's more common in children and may change into classic migraine headaches over time.
Hemiplegic migraine. You have a short period of paralysis (hemiplegia) or weakness on one side of your body. You might also feel numb or dizzy or notice vision changes. These symptoms can also be signs of a stroke, so get medical help right away if you have them.
Ocular migraine. This is also known as an ophthalmic or retinal migraine. It causes brief partial or total loss of vision in one eye. It also causes a dull ache behind the eye, which may spread to the rest of your head. Get medical help right away if you notice any vision changes.
Migraine with brainstem aura. You may have dizziness, confusion, or loss of balance before the headache. The pain may affect the back of your head. These symptoms usually start suddenly. You may also have trouble speaking, ringing in your ears, and vomiting. This type of migraine is strongly linked to hormone changes and mainly affects young adult women. Again, get these symptoms checked out by a doctor right away.
Status migrainosus. This very intense type of migraine can last more than 72 hours. The pain and nausea are so bad that you may need to go to the hospital. Sometimes, medicines or medication withdrawal can cause them.
Ophthalmoplegic migraine. This causes pain around your eye and may paralyze the muscles around it. Other symptoms include a droopy eyelid, double vision, or other vision changes. These symptoms can also be caused by pressure on the nerves behind the eye or by an aneurysm, so get immediate medical help if you have them.
Migraine Frequency
Doctors divide migraine into three levels of frequency.
Episodic migraine
This means you have an attack now and then, whether it's once a year or a couple times a week. Most people with migraine have two to four headaches a month.
High-frequency episodic migraine
You have headaches on 9-14 days per month for at least 3 months. People with high-frequency episodic migraine are more likely than others to develop chronic migraine.
Chronic migraine
You have headaches on more than 15 days of the month. Eight of those headaches include features such as:
- Moderate to intense head pain
- The pain on the side of your head (one or both)
- Pain that throbs or pulsates
- Pain that gets worse when you move
- Nausea or vomiting
- Sensitivity to light and sound
About 12% of Americans have migraine, and only about a third of those have chronic migraine.
Chronic and even high-frequency episodic migraine can be disabling. That's why it's important to work closely with your doctor on a treatment plan.
When to Call Your Doctor
See your doctor any time a headache doesn't go away or keeps coming back.
Get medical help right away if you have an intense headache and:
- A stiff neck with fever, nausea, and vomiting
- Numbness or weakness in your limbs
- Trouble speaking or slurred speech
- It happened very suddenly
- It's the first really bad headache you've ever had and keeps you from doing your daily activities
- You got it right after a head injury, exercise, or sex
- Confusion or memory loss
- It gets worse over a day
- It's the worst one you've ever had
- You have it in just one eye along with redness in that eye
- You're also having vision problems and it hurts to chew
- You're over 50 and this is the first time you've had this type of head pain
Migraine Diagnosis
Before making a diagnosis, your doctor will ask about your health history and your symptoms. It may help if you keep track of your symptoms and any triggers you've noticed. Write down or enter into a tracking app:
- What symptoms you have, including where it hurts
- How often you have them
- How long they last
- Other family members who have migraine
- All the medicines and supplements you take, even over-the-counter (OTC) ones
- Other medicines you remember taking in the past
Your doctor may order tests to rule out other things that could cause your symptoms. These may include:
- Blood tests
- Imaging tests like MRI or CT scans
- Electroencephalogram (EEG)
Migraine Severity
Some doctors use a tool called the Migraine Disability Assessment Scale (MIDAS ) to measure the intensity of attacks. To get your MIDAS score, you answer questions about your ability to participate in daily activities during your headaches. These include work or school tasks, household and family obligations, and social or recreational activities:
Grade 1. Little or no disability
Grade 2. Mild disability
Grade 3. Moderate disability
Grade 4. Serious disability
The MIDAS questionnaire also asks you to rate the pain of your headaches over the past 3 months on a scale of 1-10.
Migraine Treatment
There's no cure for migraine, but there are many ways to treat it. Some treatments stop attacks after they start, some prevent them, and some can do both.
Migraine medications
Common migraine treatments include:
OTC pain relievers. These drugs often work well for headaches. The main ingredients are often acetaminophen, aspirin, caffeine, and ibuprofen. Never give aspirin to anyone under age 19 because of the risk of Reye's syndrome.
If you use OTC pain medications too much, you can get rebound headaches or become dependent on them. If you're taking them more than 2 days a week, talk to your doctor about prescription drugs that may work better.
Celecoxib (Celebrex, Elyxyb). This is a prescription nonsteroidal anti-inflammatory drug that stops your body from making certain hormones that can cause pain when they reach high levels in your bloodstream. You take it to stop a migraine. Celebrex come as a tablet that you take once or twice a day. Elyxyb is a liquid you take by mouth once a day.
Triptans. These drugs balance the chemicals in your brain to stop the pain. You might get a pill to swallow, tablets you dissolve on your tongue, a nasal spray, or a shot. Examples include:
- Almotriptan (Axert)
- Eletriptan (Relpax)
- Rizatriptan (Maxalt)
- Sumatriptan (Imitrex)
- Zolmitriptan (Zomig)
Ditans. Lasmiditan (Reyvow) is a tablet that eases pain, nausea, and sensitivity to light or sound. It comes in pill form. It interferes with the release of CGRP.
Gepants. Your doctor might give you rimegepant (Nurtec) or ubrogepant (Ubrelvy) if other treatments don't help. You'll take them in tablet form. Another type, zavegepant (Zavzpret) comes as a nasal spray. These medications work in a different way to suppress CGRP.
Ergotamine (Cafergot, Ergomar, Migergot). This also works on the chemicals in your brain.
Nausea medicine. Your doctor can prescribe medication if you get nausea with your migraine.
Preventive medicines. If other treatments don't work, your headaches are serious, or you have four or more headache days a month, your doctor may suggest these. You take them regularly to make your headaches less painful or frequent. They include:
- Seizure medicines
- Blood pressure medicines like beta-blockers and calcium channel blockers
- Some antidepressants
Medications that help stop the action of CGRP can also work to prevent migraines. These include:
- Atogepant (Qulipta)
- Eptinezumab (Vyepti)
- Erenumab (Aimovig)
- Fremanezumab (Ajovy)
- Galcanezumab (Emgality)
Migraine devices
The FDA has approved several devices to treat or prevent migraine symptoms. They use magnetic or electrical energy to target nerves or nerve activity involved in head pain, an approach called neuromodulation. You might try them if medications don't work well for you, or use them together with medication.
The devices include:
Single-pulse transcranial magnetic stimulation (eNeura). To usethis prescription device, you place it on the back of your head. It sends a pulse of magnetic energy that affects electrical signaling in your brain, which may stop or reduce pain. It's used both to treat and prevent attacks.
External trigeminal nerve stimulation (Cefaly). This nonprescription device targets the trigeminal nerve, which provides sensation to parts of your head and face. It delivers stimulation through electrodes you place on your forehead. You can use it to treat or prevent headaches. You might also hear this type of device called a transcutaneous electrical nerve stimulation unit.
Noninvasive vagal nerve stimulator (Gammacore). This type works on your vagus nerve, a long nerve that's a key player in your nervous system. To use it, you hold it in your hand and place it against your neck. It's approved to both stop and prevent headaches and requires a prescription.
Remote electrical neuromodulator (Nerivio). You also need a prescription for this device, which you apply to your upper arm and control with a phone app. It stimulates nerves in your arm that are part of a pathway for pain signals. It's used to get rid of migraine pain once it starts.
Combined occiputal and trigeminal neurostimulation (Relivion). This headband-like device stimulates the trigeminal nerve as well as the occipital nerve that runs along the back of your head. This can help stop an attack. The device, which is available by prescription, is controlled via a phone app.
Botox for migraine
If you have chronic migraine, shots of botulinum toxin type A (Botox) can help reduce the frequency of your headaches. The toxin is thought to interrupt the pain signals in your head. You'll get several shots in your head and neck. Each treatment lasts up to 12 weeks.
Migraine surgery
Some people have surgery for migraine when other treatments don't work. But this approach hasn't been well-studied, and it's controversial. The American Headache Society doesn't recommend surgery unless it's done as part of a research study.
The two main types of migraine surgery are:
Nerve decompression surgery. In this operation, a surgeon removes tissue that's putting pressure on and irritating nerves that are thought to be trigger points for migraine.
Neurectomy. This involves cutting the end of a nerve in the area where your pain starts.
Cognitive behavioral therapy for migraine
A therapist can teach you how your actions and thoughts affect how you sense pain. They can also teach you ways to reduce stress, a known trigger for migraine attacks.
Migraine Home Remedies
You may be able to ease symptoms by:
- Resting with your eyes closed in a dark, quiet room
- Putting a cool compress or ice pack on your forehead
- Drinking plenty of liquids
- Placing a warm compress or washcloth on your head or the back of your neck
- Gently massaging your neck, scalp, or temples
- Meditating
Complementary and alternative treatments for migraine
Some people get relief with therapies they use in addition to or instead of traditional medical treatment. These are called complementary or alternative treatments. For migraine, they include:
Biofeedback. This helps you take note of stressful situations that could trigger symptoms. If the headache begins slowly, biofeedback can stop the attack before it becomes full-blown.
Supplements. Research has found that some vitamins, minerals, and herbs can prevent or treat migraines. These include riboflavin, coenzyme Q10, feverfew, magnesium, and melatonin. Butterbur may head off attacks, but it can also affect your liver enzymes.
Body work. Physical treatments like chiropractic, massage, acupressure, acupuncture, and craniosacial therapy might ease headache symptoms. Mind-body practices like yoga or tai chi might also help.
Talk to your doctor before you try any complementary or alternative treatments.
Migraine Health Disparities
Not only are women about three times more likely to have migraine than men, their headaches tend to be worse and last longer. Experts think that's mostly because hormonal changes can trigger migraines, though stress may also play a role.
Migraine is more common among people who identify as bisexual, gay, or lesbian than in those who describe themselves as straight (heterosexual). One study found that the condition affected about:
- 37% of bisexual women
- 25% of lesbians
- 23% of bisexual men
- 20% of straight women
- 15% of gay men
- 10% of straight men
Some researchers believe one reason for this could be the stress of coping with stigma and discrimination. Unequal treatment for LGBT+ people within the health-care system may also be to blame.
Researchers say the rate of migraine is similar across different racial groups in the U.S. But Hispanic people are 50% less likely to be diagnosed than White people, and Black people are 25% less likely to be diagnosed. And while 37% of White people with migraine get prescriptions for medication to stop their headaches, just 14% of Black people do.
The reasons for these differences include racism, distrust of the medical system, and a lack of minority doctors, experts say. Socioeconomic status also plays a major role. People with lower income levels are 60% more likely to have migraine. But they're much less likely to have access to high-quality health care.
Living With Migraines
Try these steps to prevent symptoms:
- Identify and avoid your triggers. Keep track of your symptom patterns in a diary or on an app so you can figure out what may be causing your attacks.
- Manage stress. Relaxation techniques like meditation, yoga, and mindful breathing can help.
- Eat and sleep on a regular schedule.
- Drink lots of fluids.
- Get plenty of rest.
- Get regular moderate exercise.
Are migraines a disability?
Not everyone with migraine is considered to have a disability under the federal Americans With Disabilities Act. It may be considered a disability if it "substantially" limits your ability to do important life activities like working, communicating, or caring for yourself.
Takeaways
Migraine is a neurological (brain and nervous system) disorder whose main symptom is intense headaches. There's no cure, but medical treatment and healthy lifestyle changes can help keep them under control. If you have headaches that interfere with your daily life, make an appointment with your doctor or a headache specialist.
Migraine FAQs
Are migraines curable?
There's no cure for migraines yet. But medications can help prevent or stop them or keep your symptoms from getting worse. You can also avoid things that trigger your migraines. Lifestyle changes like easing stress and having good sleep habits can help, too.
Are migraines fatal?
Most migraines don't cause lasting harm. Rarely, you can have a complication called migrainous infarction. That's when you have a stroke while you're having a migraine. But there's no evidence migraine can trigger a stroke.
It's extremely rare, but a hemiplegic migraine can sometimes lead to a coma or other serious complications.
A very intense headache that starts suddenly can be a sign of another, more serious condition, like a stroke or aneurysm. Get medical help right away if this happens.

