Arthritis is a general term that means inflammation of the joints. Osteoarthritis, commonly known as wear and tear arthritis, is the most common type of arthritis. It is associated with a breakdown of cartilage in joints and can occur in almost any joint in the body. It commonly occurs in the weight-bearing joints of the hips, knees, and spine. It also affects the fingers, thumb, neck, and large toe.
Osteoarthritis -- also called OA -- usually does not affect other joints unless previous injury , excessive stress or an underlying disorder of cartilage is involved.
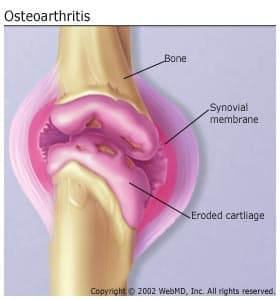
Cartilage is a firm, rubbery material that covers the ends of bones in normal joints. Its main function is to reduce friction in the joints and serve as a "shock absorber." The shock-absorbing quality of normal cartilage comes from its ability to change shape when compressed (flattened or pressed together).
Osteoarthritis causes the cartilage in a joint to become stiff and lose its elasticity, making it more susceptible to damage. Over time, the cartilage may wear away in some areas, greatly decreasing its ability to act as a shock absorber. As the cartilage deteriorates, tendons and ligaments stretch, causing pain. If the condition worsens, the bones could rub against each other.
Who Gets Osteoarthritis?
Osteoarthritis affects an estimated 27 million Americans. The chance of developing the disease increases with age. Most people over age 60 have osteoarthritis to some degree, but its severity varies. Even people in their 20s and 30s can get osteoarthritis, although there is often an underlying reason, such as joint injury or repetitive joint stress from overuse. In people over age 50, more women than men have osteoarthritis.
What Are the Symptoms of Osteoarthritis?
Symptoms of osteoarthritis most often develop gradually and include:
- Joint aching and soreness, especially with movement
- Pain after overuse or after long periods of inactivity
- Stiffness after periods of rest
- Bony enlargements in the middle and end joints of the fingers (which may or may not be painful)
- Joint swelling
What Causes Osteoarthritis?
There are several factors that increase a person's chances of developing osteoarthritis. These include:
- Heredity. Some people have an inherited defect in one of the genes responsible for making cartilage. This causes defective cartilage, which leads to more rapid deterioration of joints. People born with joint abnormalities are more likely to develop osteoarthritis, and those born with an abnormality of the spine (such as scoliosis or curvature of the spine) are more likely to develop osteoarthritis of the spine.
- Obesity.Obesity increases the risk for osteoarthritis of the knee, hip, and spine. Maintaining ideal weight or losing excess weight may help prevent osteoarthritis of these areas or decrease the rate of progression once osteoarthritis is established.
- Injury. Injuries contribute to the development of osteoarthritis. For example, athletes who have knee-related injuries may be at higher risk of developing osteoarthritis of the knee. In addition, people who have had a severe back injury may be predisposed to develop osteoarthritis of the spine. People who have had a broken bone near a joint are prone to develop osteoarthritis in that joint.
- Joint overuse. Overuse of certain joints increases the risk of developing osteoarthritis. For example, people in jobs requiring repeated bending of the knee are at increased risk for developing osteoarthritis of the knee.
- Other diseases. People with rheumatoid arthritis, the second most common type of arthritis, are more likely to develop osteoarthritis. In addition, certain rare conditions, such as iron overload or excess growth hormone, increase the chance of developing OA.
How Is Osteoarthritis Diagnosed?
The diagnosis of osteoarthritis is based on a combination of the following factors:
- Your description of symptoms
- The location and pattern of pain
- Physical exam
- X-rays
Your doctor may use X-rays to help confirm the diagnosis and make sure you don't have another type of arthritis. X-rays show how much joint damage has occurred. An MRI may be necessary to get a better look at the joint and surrounding tissues if the X-ray results do not clearly point to arthritis or another condition.
Sometimes, blood tests will be performed to determine if you have a different type of arthritis.
If fluid has accumulated in the joints, your doctor may remove some of the fluid (called joint aspiration) for examination under a microscope to rule out other diseases.
How Is Osteoarthritis Treated?
Osteoarthritis usually is treated by a combination of treatments, including exercise, weight loss if needed, medications, physical therapy with muscle strengthening exercises, hot and cold compresses to the painful joint, removal of joint fluid, injection of medications into the joint, and use of supportive devices such as crutches or canes. Surgery may be helpful to relieve pain when other treatment options have not been effective.
The type of treatment will depend on several factors, including your age, activities and occupation, overall health, medical history, location of your osteoarthritis, and severity of the condition.
How Does Weight and Exercise Impact Osteoarthritis?
Staying at your recommended weight helps prevent osteoarthritis of the knees, hips, and spine, reduces the stress on these weight-bearing joints, and reduces pain in joints already affected. Once you have osteoarthritis, losing weight also can relieve the stress and pain in your knees.
Exercise is important to improve joint movement and to strengthen the muscles that surround the joints. Gentle exercises, such as swimming or walking on flat surfaces, are recommended, because they are less stressful on your joints. Avoid activities that increase joint pain, such as jogging or high impact aerobics. Exercises that strengthen the muscles reduce pain in patients with osteoarthritis, particularly with osteoarthritis of the knee.
What Medications Are Used to Treat Osteoarthritis?
The first step with medication is often over-the-counter pain relievers as needed. These include acetaminophen (Tylenol), ibuprofen (Advil, Motrin), and naproxen (Aleve). Don't take over-the-counter medications for more than 10 days without checking with your doctor. Taking them longer than that increases the chance of side effects. If over-the-counter treatments aren't effective, your doctor may decide to prescribe a stronger anti-inflammatory drug or other medication to help ease the pain. Some medications in the form of creams, rubs, or sprays may be applied over the skin of affected areas to relieve pain. For some people with persistent pain despite these pills or creams, steroids can be injected directly into the joint. These injections can be given several times a year, though some experts believe this may ultimately accelerate joint damage.
Injections of hyaluronic acid directly into the knee joint can relieve pain in some people with osteoarthritis.
When osteoarthritis pain is severe and other treatments are not working, some doctors will give stronger pain pills, such as narcotics.
Unfortunately, none of these will reverse or slow the progression of joint damage caused by osteoarthritis.
Are There Alternative Treatments for Osteoarthritis?
While recent research has questioned their usefulness, some medical research has shown that the supplements glucosamine and chondroitin may relieve pain in some people with osteoarthritis, especially in the knee. There is no evidence that glucosamine can help rebuild cartilage. SAMe is another supplement with potential benefits for osteoarthritis. In fact, some research has shown it may be as effective an anti-inflammatory drugs. Remember to always let your doctor know about any supplements you're taking, because they can have side effects and interact with medications.
Acupuncture has also been shown to provide significant and immediate pain relief in some people with osteoarthritis.
What Supportive Devices Are Available to Help With Osteoarthritis?
Supportive or assistive devices can help to decrease pressure on the joints with osteoarthritis. Knee supports may be helpful for some people to stabilize the ligaments and tendons and decrease pain. Canes or crutches may be helpful to take pressure off certain joints.
In addition to pain relief, assistive devices improve function and prevent falls. A licensed physical therapist or other health care professional is needed to recommend what devices are best for you.
There are also many available devices to help you perform routine daily activities that may be difficult, such as housework or cooking. Ask your doctor about talking to an occupational therapist to give you ideas about which devices may help.
Is There a Surgery for Osteoarthritis?
When osteoarthritis pain is not controlled with other treatments, or when the pain prevents you from participating in your normal activities, you may want to consider surgery.
There are several types of surgery for osteoarthritis. They include:
- Arthroscopy to clean out the damaged cartilage or repair tissues. It is most commonly performed on the knee and shoulder. Recent evidence has questioned its effectiveness for osteoarthritis.
- Joint replacement surgery to replace the damaged joint with an artificial one. Joint replacement surgery should be considered when the severity of the joint pain significantly interferes with a person’s function and quality of life. Even under the best of circumstances, surgery cannot return the joint to its normal state (artificial joints do not have all of the motion of a normal joint), but movement and function are significantly improved. In addition, an artificial joint will greatly diminish pain. The two joints most often replaced are the hip and the knee. Artificial joints are now also available to replace shoulders, fingers, elbows, and ankles to treat severe pain that has not responded to other treatments.
- Joint fusion to remove the damaged joint and fuse the two bones on each side of the joint. This is done more often in areas in which joint replacement is not effective.
Talk to your doctor to determine if any of these treatment options are right for you.

