Uses
This medication is used to treat or prevent certain skin conditions related to Hansen's disease, once known as leprosy (erythema nodosum leprosum). Thalidomide is also used to treat a certain type of cancer (multiple myeloma). It works in Hansen's disease by reducing swelling and redness (inflammation). It also reduces the formation of blood vessels that feed tumors.
How to use Thalomid
Read the Medication Guide provided by your pharmacist before you start using thalidomide and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth as directed by your doctor, usually once daily at bedtime at least 1 hour after the evening meal. Swallow this medication whole with water.
Dosage is based on your medical condition and response to treatment. Do not increase your dose or take this medication more often than prescribed. Your condition will not improve any faster, and the risk of serious side effects may be increased.
Keep the capsules in their blister pack until ready to use. Do not open or break the capsules, or handle them any more than needed. If any of the powder from the capsule gets on your skin, wash the area with soap and water.
Since this drug can be absorbed through the skin and lungs and may harm an unborn baby, women who are pregnant or who may become pregnant should not handle this medication or breathe the dust from broken capsules. All people should wash their hands thoroughly after handling this drug.
This medication passes into body fluids (such as urine). Avoid contact with body fluids from people taking this drug. Wear protective clothing (such as gloves) when handling these body fluids (such as during cleanup). If contact occurs, wash skin with soap and water.
Use this medication regularly to get the most benefit from it. To help you remember, take it at the same time each day. If you are taking this medication for Hansen's disease, your skin condition may become worse when the drug is suddenly stopped. Your dose may need to be gradually decreased.
Tell your doctor if your condition does not improve or if it worsens after 2 weeks.
Side Effects
See also Warning section.
Drowsiness, dizziness, lightheadedness, constipation, weakness, and dry skin may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
To reduce the risk of dizziness and lightheadedness, get up slowly when rising from a sitting or lying position.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Thalidomide may cause possibly severe nerve damage, which may be permanent. This may occur during treatment or after treatment has stopped. Tell your doctor right away if you develop any of the following symptoms: numbness/tingling/pain/burning in the feet or hands, muscle weakness/cramps, feeling of tightness in the feet.
Tell your doctor right away if you have any serious side effects, including: mental/mood changes (such as confusion, anxiety), shaking (tremor), shortness of breath, arm/leg swelling, fast/slow heartbeat, easy bruising/bleeding, black/bloody stools, vomit that contains blood or looks like coffee grounds.
People with multiple myeloma who are treated with this medication may rarely get other cancers (such as acute leukemia). Consult your doctor for more details.
Get medical help right away if you have any very serious side effects, including: seizures.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US -
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Warnings
Women who are pregnant must not use thalidomide. Women must avoid becoming pregnant while taking this medication. Even a single dose of thalidomide has caused severe (often fatal) birth defects when used during pregnancy. You must have 2 negative pregnancy tests before you start treatment with thalidomide (within 10 to 14 days before and 24 hours before starting), repeating the test at least monthly. Do not start or continue thalidomide treatment unless you have a negative pregnancy test result.
Female patients must use 2 effective forms of birth control (or completely avoid sexual intercourse) for 1 month before starting thalidomide, during use, and for 1 month after stopping this drug. Talk to your doctor about reliable birth control choices. If your period is late, or if you have sexual intercourse at any time without using 2 effective forms of birth control, stop taking this medication and contact your doctor right away. (See also Precautions section.)
Because thalidomide also passes into semen, men who use this drug and have sex with women must use a latex condom during all sexual contact, even if they have had a vasectomy. Keep using condoms and other birth control as directed until 1 month after thalidomide treatment has been stopped.
To receive thalidomide in the United States, you must understand, agree to, and carefully follow the requirements of the REMS Program for this medication. If you live in Canada or any other country, consult your doctor and pharmacist for your country's regulations.
When used to treat a certain type of cancer (multiple myeloma), thalidomide can increase the risk of serious blood clots in the legs or lungs, as well as heart attacks and strokes. Get medical help right away if you develop shortness of breath, chest pain, jaw/neck/left arm pain, weakness on one side of your body, trouble speaking, sudden vision changes, or arm/leg swelling. While you are taking thalidomide, your doctor may also direct you to take aspirin or other "blood thinners" (such as warfarin) to lessen the risk of these types of blood clots. Talk to your doctor for more information, and tell him/her if you have diabetes, high blood pressure or high cholesterol, kidney problems, or if you smoke. (See also Side Effects section.
Precautions
Before taking thalidomide, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: certain blood disorders (low platelet/white blood cell count), numbness/tingling of arms/legs, seizures.
Caution is advised when using this drug in people with HIV because they may be more sensitive to the effects of the drug. While thalidomide is used to treat muscle wasting and other HIV-related conditions, the drug might affect the amount of HIV in your system (viral load). The manufacturer recommends having HIV tests from time to time.
This drug may make you dizzy or drowsy. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).
Since this drug can be absorbed through the skin and lungs and may harm an unborn baby, women who are pregnant or who may become pregnant should not handle this medication or breathe the dust from broken capsules.
Thalidomide must not be used during pregnancy due to the risk of severe birth defects and other serious, sometimes fatal harm to an unborn baby. If you are female and become pregnant or think you may be pregnant, if your period is late or you have unusual menstrual bleeding, or if you stop using 2 forms of birth control, stop taking thalidomide and tell your doctor right away. If you are male and have had unprotected sex with a woman who can become pregnant, or if you think your sexual partner may be pregnant, tell both of your doctors right away. (See also Warning section.)
It is unknown if this drug passes into breast milk. Because of the possible risk to the infant, breastfeeding is not recommended while using this drug. Consult your doctor before breastfeeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Tell your doctor or pharmacist if you also take drugs that cause drowsiness such as: alcohol, marijuana (cannabis), certain antihistamines (such as diphenhydramine), medicine for sleep or anxiety (such as alprazolam, diazepam, zolpidem), muscle relaxants, opioid pain relievers (such as codeine), psychiatric medicines (such as chlorpromazine, risperidone, amitriptyline, trazodone).
Check the labels on all your medicines (such as cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.
It is very important for women to use 2 forms of effective birth control while taking this medication. Some drugs may cause hormonal birth control (such as pills, patch, ring) to work less well by decreasing the amount of birth control hormones in your body. This effect can result in pregnancy. Examples include griseofulvin, modafinil, rifamycins (such as rifampin, rifabutin), ritonavir, St. John's wort, drugs used to treat seizures (such as barbiturates, carbamazepine, felbamate, phenytoin, primidone, topiramate), HIV drugs (such as nelfinavir, nevirapine), among others.
Tell your doctor when you start any new drug, and discuss if you should use reliable backup birth control while using the new drug and for 1 month after stopping the new drug.
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call 1-800-222-1222. Canada residents can call 1-844-764-7669. Symptoms of overdose may include: prolonged sleep.
Do not share this medication with others. Do not donate blood, organs, eggs, or sperm while taking thalidomide.
Lab and/or medical tests (such as pregnancy tests, white blood cell/platelet count) should be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.
If you miss a dose, take it as soon as you remember. If it is more than 12 hours after the missed dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Keep capsules in the original blister pack until ready to use. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images
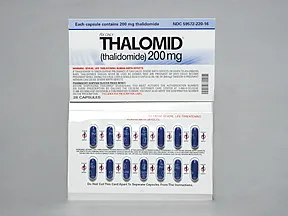
Thalomid 200 mg capsule
Color: blueShape: oblongImprint: logo CELGENE 200mgThis medicine is a blue, oblong, capsule imprinted with "logo" and "CELGENE 200mg".
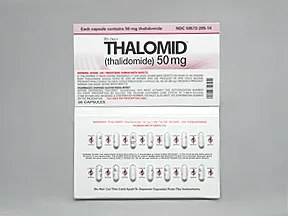
Thalomid 50 mg capsule
Color: whiteShape: oblongImprint: logo CELGENE 50 mgThis medicine is a blue, oblong, capsule imprinted with "logo" and "CELGENE 200mg".

Thalomid 100 mg capsule
Color: tanShape: oblongImprint: logo CELGENE 100mgThis medicine is a blue, oblong, capsule imprinted with "logo" and "CELGENE 200mg".
Are you currently using Thalomid?
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.