Uses
This medication is used to treat certain types of cancer (kidney, pancreas, and intestinal). It is also used to treat people who are at high risk of the kidney cancer coming back again after having kidney surgery. Sunitinib works by stopping or slowing the growth of cancer tumors. It also works by slowing the growth of new blood vessels within the tumor.
How to use Sunitinib 50 Mg Capsule Antineoplastic - Protein-Tyrosine Kinase Inhibitors
Read the Medication Guide provided by your pharmacist before you start taking sunitinib and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth with or without food as directed by your doctor, usually once daily. For some conditions, you may be directed to take this medication for a specific period of time followed by another period of time off of the drug. For other conditions, you may be directed to take sunitinib every day without a break. Follow your doctor's instructions carefully.
Drink plenty of fluids during treatment with this medication, unless otherwise directed by your doctor.
The dosage is based on your medical condition, response to treatment, and other medications you may be taking. Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Do not increase your dose or take this medication more often without your doctor's approval. Your condition will not improve any faster, and the risk of serious side effects may be increased.
Use this medication regularly in order to get the most benefit from it. To help you remember, take it at the same time each day.
Avoid eating grapefruit or drinking grapefruit juice while using this medication unless your doctor or pharmacist says you may do so safely. Grapefruit can increase the chance of side effects with this medicine. Ask your doctor or pharmacist for more details.
Side Effects
See also Warning section.
Upset stomach, nausea, vomiting, diarrhea, change in taste, decreased appetite, dry/cracked/thickened skin, watering eyes, swelling around eyes, numbness/tingling of arms/legs, or tiredness may occur. In some cases, drug therapy may be necessary to prevent or relieve nausea/vomiting/diarrhea. Eating several small meals or limiting activity may help lessen some of these effects. If these effects last or get worse, notify your doctor or pharmacist promptly.
This medication may cause patchy or complete hair loss and changes in hair/skin color. These effects are not harmful. However, talk to your doctor for more details since changes in skin color may also be a sign of a more serious condition.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Tell your doctor right away if you have any serious side effects, including: headache, rash/blisters on palms of hands/soles of feet, sores/pain on the tongue/mouth, easy bruising/bleeding, cold/heat intolerance, pain/redness/swelling of arms or legs, black/bloody stools, vomit that looks like coffee grounds, coughing up blood, slow wound healing, jaw pain, toe/joint pain, signs of low blood sugar (such as hunger, shakiness, fast heartbeat, sweating), symptoms of heart failure (such as shortness of breath, swelling ankles/feet, unusual tiredness, unusual/sudden weight gain), mental/mood changes (such as decreased alertness, irritability, nervousness), vision changes (such as decreased vision).
This medication may raise your blood pressure. Check your blood pressure regularly and tell your doctor if the results are high. Your doctor may control your blood pressure with medication.
Sunitinib sometimes causes side effects due to the rapid destruction of cancer cells (tumor lysis syndrome). To lower your risk, your doctor may add a medication and tell you to drink plenty of fluids. Tell your doctor right away if you have symptoms such as: signs of kidney problems (such as painful urination, cloudy/pink/bloody urine, change in the amount of urine), muscle spasms/weakness.
Get medical help right away if you have any very serious side effects, including: trouble breathing, chest/jaw/left arm pain, sudden/severe back pain, weakness on one side of the body, trouble speaking, severe dizziness, fainting, fast/slow/irregular heartbeat, seizures.
Sunitinib has rarely caused very serious (possibly fatal) skin reactions. Get medical help right away if you develop symptoms of serious skin reactions, including: rash, hot/peeling/blistering/painful skin, red/purple skin.
This medication may lower your ability to fight infections. This may make you more likely to get a serious (rarely fatal) infection or make any infection you have worse. Tell your doctor right away if you have any signs of infection (such as sore throat that doesn't go away, fever, chills, cough).
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US -
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Warnings
Rarely, sunitinib has caused severe (sometimes fatal) liver problems. Get medical help right away if you develop symptoms of liver problems such as nausea/vomiting that doesn't stop, severe stomach/abdominal pain, dark urine, pale stools, or yellowing eyes/skin. Your doctor will monitor for liver problems with blood tests. Tell all of your doctors and pharmacists if you have ever stopped taking sunitinib because of liver problems (including high liver enzymes).
Precautions
Before taking sunitinib, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: bleeding problems, heart problems (such as heart attack), high blood pressure, liver problems, kidney disease, thyroid disease, diabetes, blood vessel problems (such as an aneurysm or a tear/break in the aorta or other blood vessels).
Sunitinib may cause a condition that affects the heart rhythm (QT prolongation). QT prolongation can rarely cause serious (rarely fatal) fast/irregular heartbeat and other symptoms (such as severe dizziness, fainting) that need medical attention right away.
The risk of QT prolongation may be increased if you have certain medical conditions or are taking other drugs that may cause QT prolongation. Before using sunitinib, tell your doctor or pharmacist of all the drugs you take and if you have any of the following conditions: certain heart problems (heart failure, slow heartbeat, QT prolongation in the EKG), family history of certain heart problems (QT prolongation in the EKG, sudden cardiac death).
Low levels of potassium or magnesium in the blood may also increase your risk of QT prolongation. This risk may increase if you use certain drugs (such as diuretics/"water pills") or if you have conditions such as severe sweating, diarrhea, or vomiting. Talk to your doctor about using sunitinib safely.
Do not have immunizations/vaccinations without the consent of your doctor, and avoid contact with people who have recently received oral polio vaccine or flu vaccine inhaled through the nose.
To lower the chance of getting cut, bruised or injured, use caution with sharp objects like safety razors or nail cutters and avoid activities such as contact sports.
Wash your hands well to prevent the spread of infections.
Some people taking sunitinib may have serious jawbone problems. Your doctor should check your mouth before you start this medication. Tell your dentist that you are taking this medication before you have any dental work done. To help prevent jawbone problems, have regular dental exams and learn how to keep your teeth and gums healthy. If you have jaw pain, tell your doctor and dentist right away.
Before having surgery (including dental procedures), tell your doctor or dentist about this medication and all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
This medication may cause wounds to heal slowly or poorly. Your doctor or dentist may tell you to temporarily stop treatment with this medication at least 3 weeks before surgery or a dental procedure. Ask your doctor or dentist for specific instructions about when to stop and when to restart treatment with this medication. Tell your doctor/dentist right away if you have wounds that are not healing well.
Older adults may be more sensitive to the side effects of this drug, especially QT prolongation (see above).
Tell your doctor if you are pregnant or plan to become pregnant. You should not become pregnant while using sunitinib. Sunitinib may harm an unborn baby. Your doctor should order a pregnancy test before you start this medication. Women using this medication should ask about reliable forms of birth control during treatment and for 4 weeks after the last dose. Men using this medication should ask about reliable forms of birth control during treatment and for 7 weeks after the last dose. If you or your partner becomes pregnant, talk to your doctor right away about the risks and benefits of this medication.
It is unknown if this drug passes into breast milk. Because of the possible risk to the infant, breastfeeding is not recommended while using this drug and for 4 weeks after the last dose. Consult your doctor before breastfeeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug include: bevacizumab, "blood thinners" (anticoagulants such as warfarin, heparins).
Many drugs besides sunitinib may affect the heart rhythm (QT prolongation), including amiodarone, dofetilide, pimozide, procainamide, quinidine, sotalol, macrolide antibiotics (such as erythromycin), among others. Before using sunitinib, report all medications you are currently using to your doctor or pharmacist.
Other medications can affect the removal of sunitinib from your body, which may affect how sunitinib works. Examples include azole antifungals (such as ketoconazole, itraconazole), cimetidine, HIV protease inhibitors (such as saquinavir), macrolide antibiotics (such as erythromycin), ritonavir, St. John's wort, certain drugs used to treat seizures (such as phenytoin, phenobarbital), among others.
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call 1-800-222-1222. Canada residents can call 1-844-764-7669.
Do not share this medication with others.
Lab and/or medical tests (such as blood pressure, complete blood count, heart/liver/kidney/thyroid function, blood sugar, uric acid level, pancreatic enzymes, urine protein levels, blood mineral levels such as sodium, potassium, phosphate) should be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.
If you miss a dose, take it as soon as you remember. If it is less than 12 hours before the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images

sunitinib malate 50 mg capsule
Color: light brownShape: oblongImprint: TEVA 8231 TEVA 8231This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".
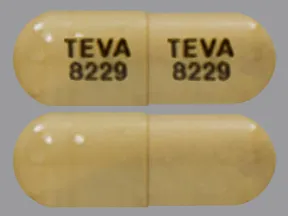
sunitinib malate 37.5 mg capsule
Color: yellowShape: oblongImprint: TEVA 8229 TEVA 8229This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".

sunitinib malate 12.5 mg capsule
Color: maroonShape: oblongImprint: TEVA 8199 TEVA 8199This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".
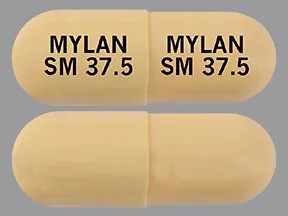
sunitinib malate 37.5 mg capsule
Color: ivoryShape: oblongImprint: MYLAN SM 37.5 MYLAN SM 37.5This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".
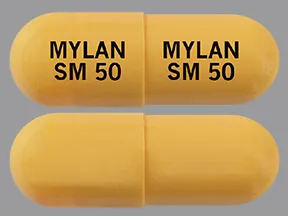
sunitinib malate 50 mg capsule
Color: yellowShape: oblongImprint: MYLAN SM 50 MYLAN SM 50This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".
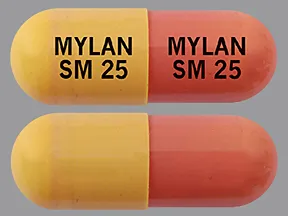
sunitinib malate 25 mg capsule
Color: yellow,pinkish-brownShape: oblongImprint: MYLAN SM 25 MYLAN SM 25This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".

sunitinib malate 12.5 mg capsule
Color: pinkish-brownShape: oblongImprint: MYLAN SM 12.5 MYLAN SM 12.5This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".

sunitinib malate 25 mg capsule
Color: maroon,light brownShape: oblongImprint: TEVA 8224 TEVA 8224This medicine is a light brown, oblong, capsule imprinted with "TEVA 8231" and "TEVA 8231".
Are you currently using Sunitinib 50 Mg Capsule Antineoplastic - Protein-Tyrosine Kinase Inhibitors?
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.