What Is Celiac Disease?
Celiac disease is an autoimmune disorder that’s triggered when you eat gluten , a protein found in wheat, barley, and other grains. The condition is also known as celiac sprue, nontropical sprue, or gluten-sensitive enteropathy.
When someone with celiac disease eats or drinks something that contains gluten, their body overreacts to the protein. This damages their villi, small finger-like projections found along the wall of the small intestine. When your villi are injured, your small intestine can’t properly absorb nutrients from food. Eventually, this can lead to malnourishment and potentially to symptoms ranging from weakened bones to mood changes to miscarriage.
Most people with celiac disease never know they have it. Researchers think that as few as 20% of people with the disease get the right diagnosis. The damage to your intestine is very slow, and the symptoms vary a lot from person to person.
Is celiac disease serious?
While celiac disease itself isn't usually life-threatening, it can lead to serious complications and symptoms if it's not managed. Untreated celiac disease also raises your risk for some types of cancer.
Celiac disease vs gluten intolerance
Celiac disease isn’t the same thing as gluten intolerance or gluten sensitivity. Gluten intolerance symptoms can resemble some of the digestive symptoms of celiac disease. But people with gluten intolerance don’t show an immune response or damage to the small intestine.
Celiac disease vs. wheat allergy
Celiac disease isn’t the same thing as a food allergy, so the symptoms are different. If you’re allergic to wheat but eat something with wheat in it, you may have itchy or watery eyes or a hard time breathing.
Celiac Disease Symptoms
Celiac disease symptoms in adults
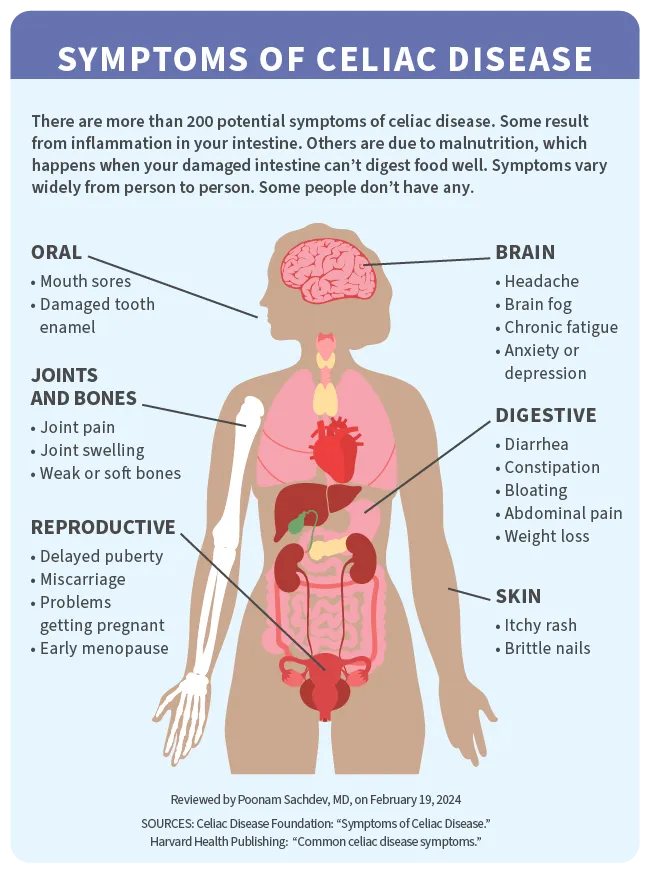
The symptoms of celiac disease vary widely from person to person. Some people don’t notice any, though the disease could still be harming their intestines.
If you do have them, symptoms can affect many parts of your body. You may notice digestive symptoms shortly after eating or drinking something that contains gluten. These could include:
- Abdominal (belly) pain
- Bloating or a feeling of fullness
- Constipation
- Diarrhea
- Gas
- Heartburn
- Nausea
- Poop that’s pale, smells especially bad, or floats (steatorrhea)
You might have other symptoms over time because your body isn't getting enough nutrients. They include:
- Anemia
- Bone or joint pain
- Itchy, blistery rash (doctors call this dermatitis herpetiformis)
- Headaches
- Fatigue
- Weakened bones
- Mood changes
- Mouth ulcers and dental problems
- Nervous system problems, including numb or tingling hands or feet, balance problems, or changes in awareness
- Reduced spleen function (hyposplenism)
- Weight loss
Celiac disease symptoms in females
Some symptoms affect women (and those assigned as female at birth) in particular. They may stem from malnutrition and/or from your body's immune reaction to gluten. They include:
- Irregular periods
- Early menopause
- Miscarriage
Untreated celiac disease has been linked to infertility (trouble getting pregnant) in both females and males.
Celiac disease symptoms in children
Children with celiac disease are more likely than adults to have intestinal problems, including:
- Bloating or belly swelling
- Constipation
- Diarrhea
- Pale, foul-smelling poop
- Upset stomach or vomiting
- Weight loss
If celiac disease keeps a child’s body from absorbing the nutrients they need, they can have problems including:
- Anemia
- Damaged tooth enamel
- Delayed puberty
- In infants, failure to thrive
- Crankiness or mood changes
- Neurological problems like learning disabilities and attention deficit hyperactivity disorder (ADHD)
- Slow growth and short height
Celiac rash (dermatitis herpetiformis)
About 1 in 4 people with celiac disease get an itchy, blistering rash. It happens more in adults than children, and more in men than women. It’s most common in these areas:
- Buttocks
- Elbows
- Knees
- Scalp
- Lower back
Celiac Disease Causes and Risk Factors
Causes of celiac disease
Celiac disease is caused by your immune system's abnormal response to gluten. Your immune system reacts to usually harmless protein as if it's a threat to your body and produces antibodies against it. This causes inflammation (swelling) in your gut, which damages your villi and leads to the symptoms of celiac disease.
It's not entirely clear why this reaction happens. Scientists believe celiac disease mostly affects people who have certain gene changes (mutations). But not everyone who has the mutation will get the disease.
Doctors think celiac disease can be triggered by things that are stressful to your body and immune system, such as a viral infection, surgery, pregnancy, or emotional trauma.
Risk factors for celiac disease
If one of your close family members has it, like a parent or sibling, you have a 1 in 10 chance of getting celiac disease. Like many immune disorders, it's more likely to affect women (and those identified as female at birth). They make up 60%-70% of those diagnosed with the condition.
The disease is also most common among white people and people who have other diseases, including:
- Hashimoto’s thyroiditis
- Type 1 diabetes
- Addison’s disease
- Down syndrome
- Rheumatoid arthritis
- Turner syndrome (a condition in which a female is missing an X chromosome)
- Multiple sclerosis (MS)
- Autoimmune hepatitis
- Sjogren’s syndrome
- Idiopathic dilated cardiomyopathy
- IgA nephropathy
- Lupus
- Irritable bowel syndrome (IBS)
- Chronic pancreatitis
- Psoriasis
- Scleroderma
- Williams syndrome
- Primary biliary cirrhosis
- Lactose intolerance
- Intestinal lymphoma
- Intestinal cancer
When does celiac disease develop?
You can get celiac disease at any age. But it's most likely to be diagnosed:
- In children between the ages of 8 months and 1 year (when they first start eating foods with gluten)
- In adults in midlife, between ages 40 and 60
Some people have celiac disease for years before they get the right diagnosis.
Celiac Disease Complications
Over time, untreated celiac disease can lead to other health problems.
Long-term malnutrition resulting from celiac disease can cause:
Weak or softened bones
Weakened tooth enamel
Delayed growth and development in children
Problems with balance and coordination
Nerve tingling and numbness
Issues with learning and attention
Continued (chronic) inflammation from untreated celiac disease could result in:
Other food intolerances, such as lactose intolerance. Lactose is a sugar found naturally in dairy products.
Sores and scarring in your intestine, which can lead to narrowing and blockages. A sore could also turn into a dangerous hole in your intestinal wall.
A compromised immune system, which makes you more vulnerable to other illnesses, including other autoimmune disorder.
Liver disease, which may develop due to abnormally high levels of liver enzymes. Several types of liver disease have been linked to celiac diseases.
- A disease of the small intestine called collagenous sprue, in which tissue called collagen builds up and forms deposits in your small intestine. This can permanently interfere with your intestine's ability to absorb nutrients.
- In rare cases, cancer. Celiac disease has been linked to a higher risk of adenocarcinoma of the small intestine, enteropathy-associated T-cell lymphoma (EATL), and non-Hodgkin’s lymphoma.
Celiac Disease Diagnosis
It can be hard to diagnose celiac disease because its symptoms are so common and look like those of many other digestive issues.
If your doctor thinks you may have it, they'll start by asking you questions about your symptoms and about your personal and family health history. They'll likely do a physical exam to see whether you have physical signs of the disease. Then they'll do lab tests to make a diagnosis.
Celiac disease tests
The main types of tests used to diagnose celiac disease are:
Blood tests. Your doctor will start by testing a small sample of your blood for antibodies to gluten (called lymphocytes). This is called serology testing. If you're on a gluten-free diet, you'll need to come off it before having this test so the results will be correct. They may also do genetic testing on your blood to see whether you have either of the two genes for celiac disease.
Biopsy. For this test, a doctor examines a sample of tissue from your small intestine under a microscope to look for damage from celiac disease. To get the sample, a specialist does an endoscopy. They insert a long, flexible tube down your throat and all the way into your intestine. On the end of the tube is a tiny camera that guides the specialist as they take the sample.
Another type of endoscopy, called a capsule endoscopy, uses a camera that you swallow in a pill. As it move through your intestine, it takes images that your doctor can examine for signs of celiac disease.
If you're diagnosed with celiac disease, your doctor will do further tests to see if you have nutrient deficiencies and other complications. This helps them see how far the condition has progressed and determine what issues they need to treat. These tests include:
- Blood tests to check other parts of your immune system.
- Intestinal fatty acid binding protein tests to show if there’s damage to the intestine.
- A complete blood count to look for anemia (low red blood cells).
- C-reactive protein tests to show if there’s inflammation.
- Metabolic panels to test liver and kidney function.
- Vitamin D, B12, and folate tests to look for deficiencies.
- Iron and ferritin tests to look for iron deficiency
If you have a rash, doctors will take a small sample of your skin to look for signs it’s caused by celiac disease. This rash is easy to confuse with other skin problems.
Celiac Disease Stages
Doctors use what's called the Marsh Score system to describe how much damage you have to your small intestine. The score is based on what a doctor sees in your intestinal tissue sample during a biopsy.
Stage 0: Healthy with no sign of celiac disease.
Stage 1: There are more antibodies to gluten (lymphocytes) in your intestinal lining than normal. This could mean you have celiac disease and are following a gluten-free diet. It might also mean you have another condition, like a food intolerance or inflammatory bowel disease, that raises your level of lymphocytes.
Stage 2: You have more gluten antibodies than normal along with what's called crypt hyperplasia. Crypts are grooves between the villi in your intestines. Hyperplasia means these grooves are longer than they should be. This stage is rare and may mean you have a rash related to celiac disease (dermatitis herpetiformis).
Stage 3. You have more lymphocytes and longer crypts than normal, and the villi in your intestine look flattened and shrunken (villous atrophy). This stage usually means a diagnosis of celiac disease. Stage 3 is broken down further into 3a, 3b, and 3c based on the extent of damage to your villi.
Types of Celiac Disease
Nonresponsive celiac disease
If your condition doesn't improve when you follow what you think is a gluten-free diet, this is called nonresponsive celiac disease. Most often, you're being exposed to gluten without realizing it.
You could also have another issue, such as:
- Another intestinal condition, such as irritable bowel syndrome (IBS) or microscopic colitis
- Trouble digesting some types of sugar, such as lactose (the type found in dairy products) or fructose (the type found in fruit)
- Too much bacteria in your small intestine
Refractory celiac disease
If you follow a strict gluten-free diet for 6 months to a year and still have signs of celiac disease, you could have refractory celiac disease. This rare type affects only 1% to 2% of people with celiac disease, and they're almost always age 50 or older. If your condition doesn't respond to a gluten-free diet, your doctor will look for other issues that could be causing your symptoms. There's no standard treatment for refractory celiac disease, so you'll need to be under the care of a specialist. .
Celiac Disease Treatment
The most important way to treat celiac disease is to adopt a gluten-free diet for the rest of your life. After you’ve been on the diet for a few weeks, your small intestine should begin to heal, and you’ll start to feel better.
Celiac disease diet
Even small amounts of gluten can be harmful. So you'll need to avoid all foods and beverages that contain or are made with wheat, rye or barley. That means reading the labels of any prepared or processed foods you buy and checking restaurant menus (or asking servers) to see what foods are gluten-free. Sometimes even foods that don't naturally contain gluten are prepared or packaged in facilities that expose them to it.
Instead of bread, pasta, cereal, and other starches made with wheat, rye, or barley, choose:
- Rice
- Potatoes and sweet potatoes
- Corn and popcorn
- Beans
- Nuts
- Oats
- Millet
- Quinoa
- Amaranth
- Pastas, breads, and cereals that are labeled gluten-free
In place of beer, ale, or stout, choose:
- Wine
- Hard cider
- Gluten-free beer
- Gluten free liquors like light rum, potato vodka, gin, and tequila
Also:
- Choose fresh, frozen, or canned fruits and vegetables free of breading and sauces.
- Buy meats, fish, and poultry without added ingredients instead of deli meats or sausage.
- Avoid dairy products with fillers or additives (this includes some low-fat and fat-free dairy products as well as some ice cream).
- Opt for homemade soups, salad dressings, and desserts.
Sometimes you'll find gluten where you might not expect it, such as in:
- Soy sauce
- Malt vinegar
- Bouillon cubes
- Blue cheese
- Pudding mix
Common products like medications and toothpastes can also contain gluten, so it’s important to check labels.
A dietitian can help you design and stick to a healthy gluten-free diet.
Celiac disease medications
Your doctor might also prescribe:
- Corticosteroids to treat any inflammation that remains after you cut gluten from your diet, or for refractory celiac disease
- Medication, such as dapsone, to treat the rash that may result from celiac disease
- Vitamin or mineral supplements if you have anemia or serious nutritional deficiencies
When to See the Doctor for Follow-Ups
You can expect to have a follow-up appointment with your doctor 3-6 months after you're diagnosed with celiac disease. They'll check on your symptoms, determine whether you have any nutritional deficiencies, and will likely do a blood test to check the level of celiac antibodies.
You'll have another appointment 1 year after diagnosis. Your doctor will repeat the blood test along with any other tests they think you need. The goal at this point is to have almost no lymphocytes in your blood.
After that, you'll have regular annual appointments to see how well your gluten-free diet is working and check whether you have signs of any complications.
Takeaways
Celiac disease is an autoimmune disease in which your body reacts abnormally to the gluten found in wheat, rye, and barley. It's often hard to diagnose because it can cause a wide range of symptoms -- or no symptoms at all. But it can lead to serious complications. The main treatment is to follow a strict gluten-free diet for the rest of your life.
Celiac Disease FAQs
What is the prognosis for celiac disease?
The damage from celiac disease is reversible. If you cut gluten out of your diet, you'll most likely stop having symptoms, and your intestine will heal. Only about 1% to 2% of those with celiac disease don't respond to a gluten-free diet.
What is the life expectancy of someone with celiac disease?
Celiac disease itself is not fatal. Some research has found that people who have the condition are at slightly higher risk of dying early. But the reason for this isn't clear.
